Why is plasma needed?

Pictures: Unsplash von CoWomen, Christian Bruno, Alexandre Debieve, Mariana Proenca, Steve Johnson, NASA
The deposition of thin, nanostructured layers is one of the key tasks of our modern production technology. Many everyday products, such as electronic devices, PET bottles, functional surfaces, solar cells, space technology, eyeglasses coating, lenses or large architectural glasses require
such process technology.
Etching and coating processes are used to structure materials on a microscopic level. This enables functional coatings to repel water, for special robustness or compatibility with biological organisms as well as decontamination of surfaces or improved gas barriers in PET bottles for product protection. The processes used are mostly plasma-assisted, technically demanding, physically and chemically complex, and offer the user a wide range at least theoretically many degrees of freedom.
“Without this technology, we would be stuck in the 1970s listening through tinny headphones to disco music on our “small” portable cassette tape player. Carrying laptops around would be more for fitness than for convenience and mobile “smart” phones would require wheels.“
Plasma etching: “Yesterday, today, and tomorrow”, Journal of Vacuum Science & Technology A 31, 050825, 2013
write Donnelly and Kornblit of the University of Houston in 2013 to emphasize the importance of plasma process technology, especially for etching applications in microsystems technology.
But what is a plasma?
In fact, plasmas surround humans. Above all, matter in space is in the so-called plasma state, which is often defined as the fourth state of matter (besides solid, liquid and gaseous states). It describes a gas that is ionized to a certain degree. Free charged particles (electrons, ions) can be transported in fields, which defines a current. Examples of naturally occurring plasmas are for instance the sun, lightnings and northern lights.
Free electrons in space, which are released from atoms or molecules by cosmic radiation, for example, are accelerated by electric fields and collide with heavy gas particles. Elastic collisions change the momentum of the particles. Inelastic collisions, on the other hand, change the internal energy of mainly heavy particles. For instance, dissociation, excitation and ionization take place. During ionization, pairs of negatively charged electrons and positive ions are formed by releasing an electron from the atomic body of the neutral particle.
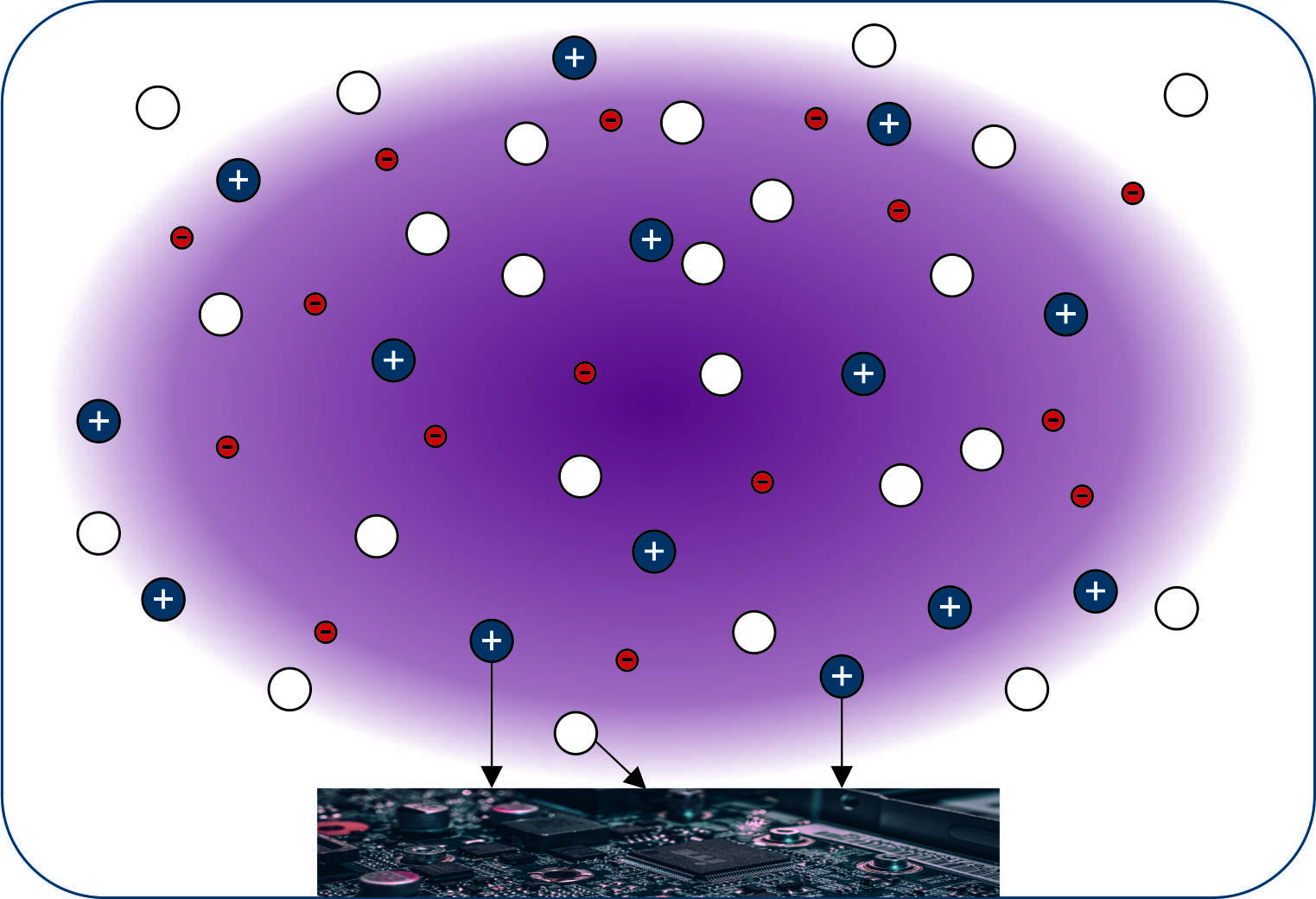
Application example: Sputter deposition
For more than 100 years, sputtering processes have been known for their use in thin film deposition. These layers are urgently needed in various fields such as the optical industry. Different sputtering technologies and a variety of measurement techniques have been developed over the centuries. Sputtering is classified as a physical vapor deposition (PVD) technique. In this process, a solid body is treated as a target by a plasma in low pressure. High energy ions, e.g. argon ions, which hit this target break surface bonds and knock single atoms out of the solid. These particles condense on surfaces, for example on a substrate. First publications on these processes date back to the 19th century. Common processes are (pulsed) direct current or medium frequency magnetron plasmas. In the 1930s, the large field of sputter coating was expanded to include roll-to-roll processes on flexible substrates. At the beginning of the second half of the 20th century with help of better models new and modern concepts for high frequency (HF) capacitively coupled plasma (CCP) were developed. Especially the use of rotating magnetrons in the early 1980s enabled a tunable asymmetrical magnetron sputtering with higher ionization efficiency and even later pulsed High-power pulsed magnetron sputtering (HPPMS).
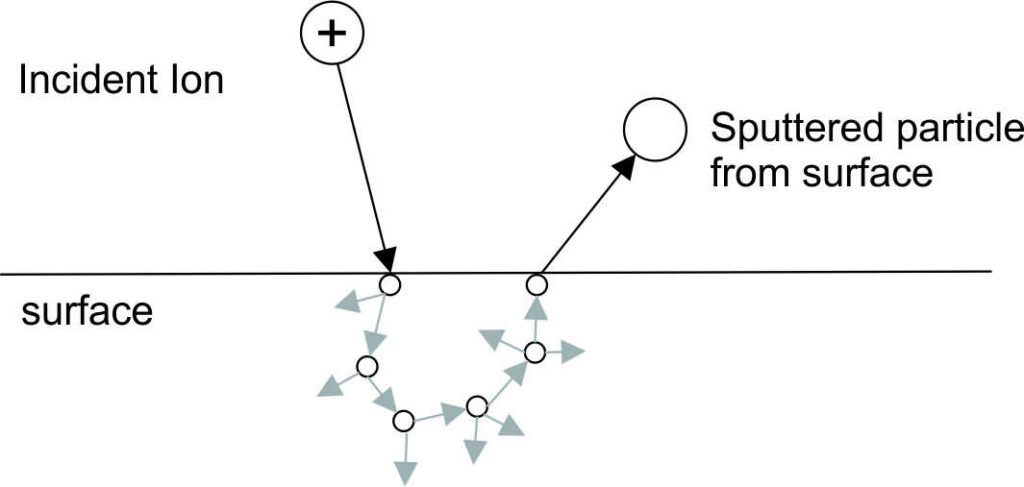
The beginning of “reactive sputtering” first appeared in 1953. By using additional reactive gas, it is possible to deposit a ceramic composite layer using a metallic target surface. For example, oxygen reacts with sputtered aluminum to form aluminum oxide. The mechanisms altered by the target oxidation, for example the secondary electron emission and the sputter yield, are still the subject of research. At least eight Nobel Prize winners in physics and chemistry had a major influence on the development of modern sputter coating, for example Joseph Thomson in 1906 for his discovery of the electron or Irving Langmuir in 1932 for his work on surface chemistry.